EN
Intended use
Medical Purpose: This product is intended to be used for inhaling medication for
respiratory disorders.
Intended User:
• Legally certified medical experts, suchas doctor, nurse and therapist.
• Caregiver or patient under the guidance of qualified medical experts for home
treatment.
• The user should also be capable of understanding general operation of
C101 Essential / X101 Easy and the content of this instruction manual.
Intended Patients: Persons who suffer from respiratory disorders and require
inhalation of medications.
Environment: This product is intended for use in a general household.
Durable period: Durable periods are as follows, provided the product is used to
nebulize 3 times a day for 10 minutes each time at room temperature (23°C). Durable
period may vary depending on usage environment.
Compressor (Main unit): 5 years
Air Tube - Nebulizer Kit - Mouthpiece - Child Mask - Adult Mask - Nosepiece -
Adapter: 1 year; Air Filter: 70 applications
Frequent usage of the product may shorten the durable period.
Know your unit
Compressor (Main Unit)
Power Switch
Air Tube
Connector
Air Filter Cover
Including air filter
Nebulizer Kit
Holder
Ventilation Slots
Rear View
Power Cord
Ventilation Slots
Power Plug
Nebulizer Kit
Air Tube Connector
Inhalation Top
Vaporiser Head
Medication Tank
Nozzle
Mouthpiece
Instruction Manual
Adapter
Nosepiece
Adult Mask (PVC)
Child Mask (PVC)
How the nebulizer kit works
The medication that is pumped up through the
medication channel is mixed with compressed air which
is generated by a compressor pump. The compressed
air mixed with medication is turned into fine particles and
sprayed when in contact with the baffle.
Important safety instructions
Read the instructions carefully before use.
Warning
1. Use the device only as inhalator for therapeutic purposes. Any other use is improper
and may be dangerous.The manufacturer is not liable for improper uses.
2. Do not use in anaesthetic or ventilator breathing circuits.
3. Always disconnect the power plug after use.
4. Do not to cover the ventilation slots during use.The compressor could get hot and
there is a possible risk of a burn if touched.
5. The compressor and power plug are not waterproof. Do not spill water or other liquids
on these parts. If liquid does spill on these parts, immediately unplug the power plug
and wipe off the liquid.
6. Do not attempt to repair the device. See Troubleshooting section.
7. Keep the device out of reach of unsupervised infants and children. The device may
contain small pieces that can be swallowed.
8. Provide close supervision when this device is used by, on, or near children or
invalids.
9. For type, dose, and regime of medication follow the instructions of your doctor or
respiratory therapist.
10. This product should not be used on patients, who are unconscious or are not
breathing spontaneously.
11. After finishing the treatment remember to disconnect the air tube from the nebulizer
and from the compressor.
12. In view of their length, the power plug and air tube could constitute a strangulation
hazard.
13. Be sure to use the compressor at a place where the power plug is easily accessible
during treatment.
14. If you experience any allergic reactions or other difficulties during use, stop using the
device immediately and consult your doctor.
15. While using this device, make sure that no mobile phone or any other electrical
devices that emit electromagnetic fields is within 30cm. This may result in degradation
of performance of the device.
How to use
1. Make sure that the power switch is in the off ( ) position.
2. Plug the power plug into a power outlet.
Note: Do not place the device in a location where it is difficult to
disconnect the power cord.
3. Remove the inhalation top from the medication tank.
1) Rotate the inhalation top anti-clockwise.
2) Lift the inhalation top out of the medication tank.
4. Add the correct amount of prescribed medication into the
medication tank.
5. Verify the presence of the vaporiser head inside the
medication tank.
6. Put the inhalation top back onto the medication tank.
1) Lower the inhalation top on the medication tank.
2) Rotate the inhalation top clockwise.
7. Attach the adapter, and the mask, mouthpiece or
nosepiece to the nebulizer kit tightly.
8. Attach the air tube. While twisting the air tube plug slightly,
push it firmly into the air tube connector.
9. Hold the nebulizer kit as indicated on the
right.
Follow the instructions of your doctor or
respiratory therapist.
Caution:
Do not tilt the nebulizer kit at an angle of greater than 30 degrees in all directions. Medication may
flow into the mouth or it may result in ineffective nebulization.
10. Turn the power switch to the on ( ) position. As the compressor starts, nebulization
begins and aerosol is generated. Gently inhale the medication. Exhale through the
nebulizer kit.
11. When treatment is completed, turn the power off and unplug the compressor from the
power outlet.
Baffle
Aerosol
Medication
Aerosol
Nozzle
Medication
Compressed Air
1
2
Troubleshooting
In case of any of the below problems occur during use, first check that no other electrical
device is within 30 cm. If the problem persists, please refer to the below.
The device does not switch on
• Check that the power plug is properly fitted to the power outlet.
• Make sure the power switch is in the on position ( ).
The device switches on but does not nebulize
• Make sure the vaporizer head is fitted in the nebulizer kit.
• Make sure the air tube is not squashed or crooked.
• Check the air filter for blockage and dirt. Replace if necessary.
• Check that sufficient amount of medication has been put into the nebulizer kit.
The device suddenly stops working during operation.
• The thermal cut-out has shut the device down for one of the following reasons:
- the device was working in an environment with temperatures higher than 40°C;
- the ventilation slots were covered.
Do not attempt to repair the device. Do not open and/or tamper with the device. No parts
of the device are user serviceable. Return the device to an authorized OMRON retail
outlet or distributor.
Please report to the manufacturer and the competent authority of the Member State in
which you are established about any serious incident that has occurred in relation to this
device.
Warranty
Thank you for buying an OMRON product. This product is constructed of high quality
materials and great care has been taken in its manufacturing. It is designed to give you the
highest level of comfort, provided it is properly operated and maintained as described in
the instruction manual.This product is guaranteed by OMRON for a period of 3 years after
the date of purchase.
The proper construction, workmanship and materials of this product is guaranteed by
OMRON. During this period of guarantee OMRON will, without charge for labour or parts,
repair or replace the defect product or any defective parts. The guarantee does not cover
any of the following:
a. Transport costs and risks of transport.
b. Costs for repairs and / or defects resulting from repairs done by unauthorized persons.
c. Periodic check-ups and maintenance.
d. Failure or wear of optional parts or other attachments other than the main device itself,
unless explicitly guaranteed above.
e. Costs arising due to non-acceptance of a claim (those will be charged for).
f. Damages of any kind including personal caused accidentally or from misuse.
Should guarantee service be required please apply to the dealer whom the product was
purchased from or an authorised OMRON distributor. For the address refer to the product
packaging / literature or to your specialised retailer.
Repair or replacement under the guarantee does not give rise to any extension or
renewal of the guarantee period.
The guarantee will be granted only if the complete product is returned together with the
original invoice / cash ticket issued to the consumer by the retailer. OMRON reserves the
right to refuse the guarantee service if any unclear information has been given.
Optional Medical Accessories
Product Description Model
Nebulizer Accessory Set
(Contents: Nebulizer Kit, Adapter, Mouthpiece, Nosepiece,
Adult Mask (PVC), Child Mask (PVC), Air Tube, Air Filter)
NEB-ASKIT-11
Nasal Shower NEB6014
Other Optional/Replacement Parts
Product Description Model
Air Filter Set (Contents: 3 pieces) 3AC408
Important information regarding Electro Magnetic Compatibility (EMC)
This device conforms to EN60601-1-2:2015 Electro Magnetic Compatibility (EMC)
standard. Further documentation in accordance with this EMC standard is available at
OMRON HEALTHCARE EUROPE at the address mentioned in this instruction manual or
at www.omron-healthcare.com.
DISPOSAL PROCEDURE (Dir. 2012/19/EU-WEEE)
This product is not to be treated as regular household waste but must be
returned to a collection point for recycling electric and electronic devices.
Further information is available from your municipality, your municipality’s
waste disposal services, or the retailer where you purchased your product.
Manufacturer 3A HEALTH CARE S.r.l.
Via Marziale Cerutti, 90F/G
25017 Lonato del Garda (BS)
Italy
Distributor OMRON HEALTHCARE EUROPE B.V.
Scorpius 33, 2132 LR Hoofddorp, THE NETHERLANDS
www.omron-healthcare.com
Subsidiaries OMRON HEALTHCARE UK LTD.
Opal Drive, Fox Milne, Milton Keynes, MK15 9DG, UK
www.omron-healthcare.com
OMRON MEDIZINTECHNIK HANDELSGESELLSCHAFT mbH
Konrad-Zuse-Ring 28, 68163 Mannheim, GERMANY
www.omron-healthcare.com
OMRON SANTÉ FRANCE SAS
3, Parvis de la Gare, 94130 Nogent-sur-Marne, FRANCE
www.omron-healthcare.com
Made in Italy
1
2
Cleaning and daily disinfecting
Carefully wash your hands before cleaning and disinfecting the nebulizer parts. Clean
the parts after each use to remove residual medication. This will prevent inefficient
nebulization and reduce risk of infection.
• Cleaning the Nebulizer Kit, Mask, Mouthpiece, Nosepiece and Adapter:
Wash them in warm water and mild, neutral detergent. Rinse them thoroughly with
clean hot tap water, gently tap to remove excess water and allow to air dry in a clean
place. It is advisable to replace the Nebulizer Kit after some 100 to 120 treatments or
after about 20 boiling cycles.
• Cleaning the Compressor and Air Tube:
Firstly, make sure that the power plug is unplugged from the power outlet. Wipe clean
with a soft cloth moistened with water or mild, neutral detergent.
• Disinfecting the Nebulizer Kit, Mask, Mouthpiece, Nosepiece and Adapter:
Always disinfect the parts before using the product for the first time and after the
product has not been used for an extended period of time as well as after the last
treatment of the day. If the parts are heavily stained, replace them with new ones. The
Nebulizer Kit, Mask, Mouthpiece, Nosepiece and Adapter can be disinfected by using
a chemical disinfectant such as ethanol, sodium hypochlorite (Milton), Quaternary
ammonium (Osvan), Chlorhexidine (Hibitane) and Amphoteric Surfactant (Tego),
following product instructions and rinsing off with clean warm water. Leave to air-dry.
Note: Never clean with benzene, thinner or a flammable chemical.
• Boiling:
The Nebulizer Kit, Mouthpiece, Nosepiece and Adapter can also be disinfected
by boiling between 15 to 20 minutes in plenty of water. After boiling, carefully remove
the parts, shake off excess water and allow to air-dry in a clean environment.
Note: Do not boil the Mask and the Air Tube.
Replacing the Air Filter:
If the air filter has changed colour, or has been used for more than
70 applications, replace it with a new one. To purchase the filters – see
Other Optional/Replacement Parts paragraph.
Remove the air filter cover using a flat screwdriver as shown in figure;
remove the filter and fit the new filter as shown.
Put the air filter cover back in place.
Note: Do not wash or clean the air filter. If the air filter Becomes wet,
replace it. Damp air filters can cause blockages.
Technical data
Product Category: Nebulizers
Product Description: Compressor Nebulizer
Model (code): C101 Essential (NE-C101-E) /
X101 Easy (NE-C101-EO)
Rating: 230V ~50Hz, (fuse: T1.6AL250V)
Power Consumption: 150VA
Operating Mode: Continuous Use
Operating Temperature/
Humidity/Air Pressure:
+5°C to +40°C/ 15% to 85%RH /
700 to 1060 hPa
Storage and Transport
Temperature/Humidity/
Air Pressure:
-20°C to +60°C / 5% to 95%RH /
500 to 1060 hPa
Weight: Approx. 1.05kg (compressor only)
Dimensions: Approx. 145 (W) × 124 (H) × 182 (D) mm
Classifications: Type BF (Applied part): Mouthpiece, Nosepiece and
Masks
IP21 (Ingress Protection)
Contents: Compressor, Nebulizer Kit, Air Tube (PVC, 100cm),
Mouthpiece, Nosepiece, Adapter, Adult Mask (PVC),
Child Mask (PVC), Instruction Manual
= Class ll
equipment
= Type BF
applied part
IP21 = Ingress
Protection
= Power off
=
Serial
number
=
Consult the
instructions
for use
=
Alternating
current = Power on
= Medical
Device
= Date of
Manufacture
Appropriate Medication
Quantities:
2ml minimum - 12ml maximum
Residual volume of
medication:
Approx. 0.7ml
Sound:Noise level
(at 1m distance):
Approx. 58dB
Particle Size (MMAD): $SSUR[ȝP
Nebulization Rate
(by weight loss):
Approx. 0.35ml/min (NaCl 0.9%)
Aerosol Output
(2ml, 1%NaF):
Approx. 0.25ml
Aerosol Output Rate
(2ml, 1%NaF):
Approx. 0.07ml/min
MMAD = Mass Median Aerodynamic Diameter
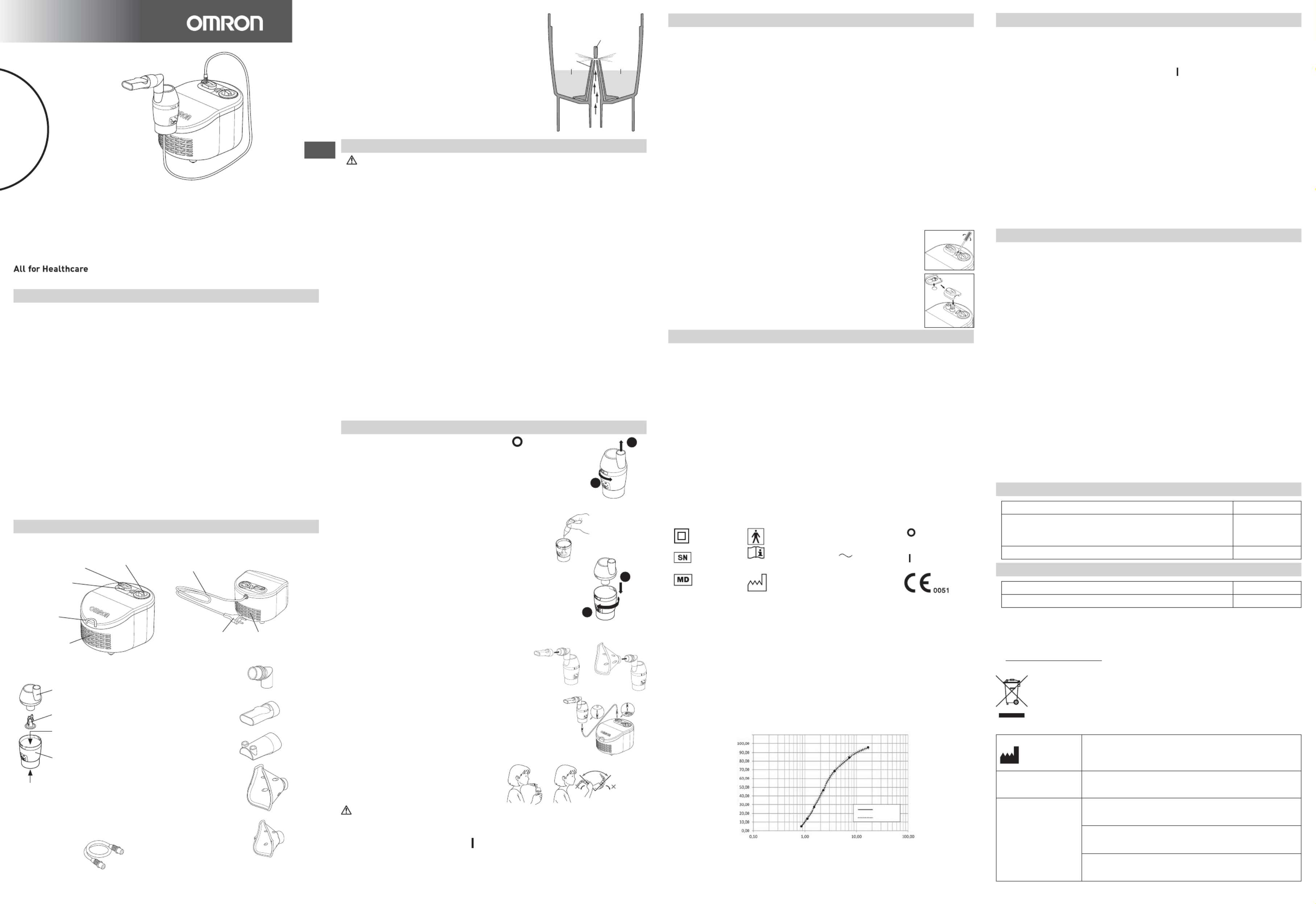
Result of cascade impactor measurements for particle size
Cumulative % particle mass of sodium fluoride undersize
3DUWLFOHVL]H'SȝP
Cumulative Undersize %
Mean
Indivisual
Performance may vary with drugs such as suspensions or high viscosity. See drug
supplier’s data sheet for further details.
Compressor Nebulizer
C101 Essential (NE-C101-E)
X101 Easy (NE-C101-EO)
Instruction Manual
IM-NE-C101-E-EN-03-11/2019
3A3621 rev.02
Issue date: 2019-11-15
Air Tube
(PVC, 100cm)