Überprüfen Sie den Lieferumfang auf äußere Unversehrtheit der Kartonverpackung und auf die Vollstän-
digkeit des Inhalts. Vor dem Gebrauch ist sicherzustellen, dass das Gerät und Zubehör keine sichtbaren
Schäden aufweisen und jegliches Verpackungsmaterial entfernt wird. Benutzen Sie es im Zweifelsfall
nicht und wenden Sie sich an Ihren Händler oder an die angegebene Kundendienstadresse.
Pulsoximeter, 2 x 1,5V AAA Batterien, Umhängeband, Gürteltasche, Schraubendreher, Gebrauchsan-
Auf dem Gerät, in der Gebrauchsanweisung, auf der Verpackung und auf dem Typschild des Geräts
werden folgende Symbole verwendet:
Bezeichnet eine möglicherweise drohende Gefahr. Wenn sie nicht gemieden wird, können Tod oder
schwerste Verletzungen die Folge sein.
Bezeichnet eine möglicherweise drohende Gefahr. Wenn sie nicht gemieden wird, können leichte
oder geringfügige Verletzungen die Folge sein.
Bezeichnet eine möglicherweise schädliche Situation. Wenn sie nicht gemieden wird, kann das
Gerät oder etwas in seiner Umgebung beschädigt werden.
Hinweis auf wichtige Informationen
Vor Beginn der Arbeit und /oder dem
Bedienen von Geräten oder Maschi-
(Elektro-)Gerät darf nicht über den
Schadstohaltige Batterien nicht im
Hersteller Herstellungsdatum
Dieses Produkt erfüllt die Anforde-
rungen der geltenden europäischen
und nationalen Richtlinien.
Verpackungskomponenten trennen
und entsprechend der kommunalen
Kennzeichnung zur Identifikation
des Verpackungsmaterials.
A = Materialabkürzung, B = Materi-
alnummer: 1-7 = Kunststoe,
Produkt und Verpackungskomponen-
ten trennen und entsprechend der
kommunalen Vorschriften entsorgen.
Gerät geschützt gegen Fremdkörper
≥12,5 mm und gegen schräges
Seriennummer Medizinprodukt
Temperaturbereich Feuchtigkeitsbereich
Pulsfrequenz (Pulsschläge pro
Arterielle Sauerstosättigung des
Alarmunterdrückung Typennummer
Chargenbezeichnung Importeur
United Kingdom Conformity As-
Unique Device Identifier (UDI)
Kennung zur eindeutigen Produkti-
BESTIMMUNGSGEMÄSSER GEBRAUCH
Das Pulsoximeter dient der nichtinvasiven Messung der arteriellen Sauerstosättigung (SpO
Herzfrequenz (Pulsfrequenz) zuhause und in Krankenhäusern (nicht in AP und APG Räumen). Dieses
Gerät ist nicht dazu geeignet eine Langzeitmessung durchzuführen.
Das Pulsoximeter ist für Personen ab einem Jahr mit und ohne Vorerkrankungen geeignet, bei welchen
die arterielle Sauerstosättigung (SpO
) und die Herzfrequenz (Pulsfrequenz) gemessen werden sollen.
Es ist für Kinder konzipiert, deren Körpergewicht zwischen 10 - 40kg beträgt, deren Fingerspitze eine
Breite von 7-11mm und eine Länge von mindestens 30mm aufweist und bei denen keine Kontraindika-
tionen vorliegen. Für Personen unter 12 Jahren ist die Anwendung nur unter Aufsicht geeignet.
Das Mindestalter für die Anwendung ist von dem Gewicht und der Fingergröße des Kindes abhängig,
weshalb die Anwendung gegebenenfalls schon früher oder erst später möglich ist.
Das Pulsoximeter eignet sich insbesondere für Risikopatienten und Kinder mit Erkrankungen des Herz-
Kreislauf-Systems, Atemwegserkrankungen oder Schlafapnoe. Ebenso ist das Pulsoximeter für Kinder
geeignet, die Symptome einer erniedrigten Sauerstosättigung zeigen (z.B. Atemnot, Herzfrequenzer-
höhung, Leistungsabfall, Nervosität oder Schweißausbrüche).
Das Pulsoximeter bietet die Möglichkeit der schnellen und einfachen Ermittlung des Sauerstosätti-
gungswerts sowie eine einfache Feststellung eines erniedrigten Sauerstosättigungswerts.
Verwenden Sie das Pulsoximeter
- NICHT, wenn Sie allergisch auf Gummiprodukte reagieren.
- NICHT, wenn das Gerät oder der Anwendungsfinger feucht ist.
- NICHT an Säuglingen < 1 Jahr.
- NICHT an Fingern mit anatomischen Veränderungen, Ödemen, Narben oder Verbrennungen.
- NICHT an Fingern mit großer Fingerdicke, die nicht zwanglos in das Gerät einführbar sind
(Fingerspitze: Breite ca. >11 mm).
- ICHT an Fingern mit zu geringer Breite und Länge (Breite ca. <7 mm, Länge ca. <30 mm). N
- NICHT an Patienten, die am Anwendungsort unruhig sind (z.B. Zittern).
Unerwünschte Nebenwirkungen
• Fingerverletzungen als chemische oder thermische Verbrennungen, Hautbräunung, Druckerosion,
sensorischer Verlust, Gangrän
• Mechanismen dieser Komplikationen können sein: Druckischämie, längerer Gebrauch, Überhitzung
der Sonde, unsachgemäße Verwendung der Sonde, Kurzschluss
• Mögliche Messabweichung an einem beeinträchtigten Finger. In diesem Fall wird der SpO
• Geringe Genauigkeit der SpO
-Messung bei kritisch kranken Patienten: Inhärenter potenzieller Feh-
ler von 3-4 % bei Messungen, die an diesen Patienten durchgeführt werden.
WARN- UND SICHERHEITSHINWEISE
Lesen Sie diese Gebrauchsanweisung sorgfältig! Ein Nichtbeachten der nachfolgenden Hinweise kann
Personen- oder Sachschäden verursachen.
• Überprüfen Sie, ob alle im Lieferumfang angegebenen Teile enthalten sind.
• Überprüfen Sie das Pulsoximeter regelmäßig, um sicherzustellen, dass das Gerät vor dem Ge-
brauch keine sichtbaren Schäden aufweist. Benutzen Sie es im Zweifelsfall nicht und wenden Sie
sich an den Beurer-Kundendienst oder an einen autorisierten Händler.
• Benutzen Sie keine Zusatzteile, die nicht vom Hersteller empfohlen, bzw. als Zubehör angeboten
• Sie dürfen das Gerät keinesfalls önen oder reparieren, da sonst eine einwandfreie Funktion nicht
gewährleistet werden kann. Bei Nichtbeachten erlischt die Garantie. Wenden Sie sich bei Repara-
turen an den Beurer-Kundendienst oder an einen autorisierten Händler.
• Wenn die Batterieanzeige auf dem Display einen niedrigen Batteriestand anzeigt, müssen die Bat-
terien ausgetauscht werden.
• Bei Personen mit Durchblutungsstörungen kann eine längere Benutzung des Pulsoximeters zu
Schmerzen führen. Verwenden Sie daher das Pulsoximeter nicht länger als 2 Stunden an einem
• Das Pulsoximeter zeigt jeweils einen momentanen Messwert, kann aber nicht für eine kontinuier-
liche Überwachung verwendet werden. Das Gerät ist so kalibriert, dass es die funktionelle Sauer-
• Das Pulsoximeter verfügt über keine Alarmfunktion und eignet sich daher nicht zur Bewertung
medizinischer Ergebnisse.
• Führen Sie aufgrund der Messergebnisse keine Selbstdiagnose oder -behandlung ohne Rückspra-
che mit Ihrem behandelnden Arzt durch. Setzen Sie insbesondere nicht eigenmächtig eine neue
Medikation an und führen Sie keine Änderungen in Art und/oder Dosierung einer bestehenden
• Eine chronische und bekannte erniedrigte Sauerstosättigung benötigt eine Überwachung durch
Ihr Pulsoximeter unter ärztlicher Kontrolle. Eine akut erniedrigte Sauerstosättigung, mit oder ohne
Begleitsymptome, ist sofort ärztlich abzuklären. Es kann sich dabei um eine lebensbedrohliche
• Schauen Sie während des Messvorgangs nicht direkt in das Gehäuseinnere. Das Rotlicht und das
unsichtbare Infrarot-Licht des Pulsoximeters sind schädlich für die Augen.
• Dieses Gerät ist nicht dafür bestimmt, durch Personen (einschließlich Kinder) mit eingeschränkten
physischen, sensorischen oder geistigen Fähigkeiten oder mangels Erfahrung und/oder mangels
Wissen benutzt zu werden, es sei denn, sie werden durch eine für Ihre Sicherheit zuständige Person
beaufsichtigt oder erhielten von ihr Anweisungen, wie das Gerät zu benutzen ist. Kinder sollten
beaufsichtigt werden, damit sie nicht mit dem Gerät spielen.
• Halten Sie Kinder vom Verpackungsmaterial fern (Erstickungsgefahr).
• Die Anzeige der Pulswelle sowie der Pulssäule, erlauben keine Abschätzung über die Puls- oder
Durchblutungsstärke am Messort, sondern dienen ausschließlich der Darstellung der aktuellen opti-
schen Signalvariation am Messort, sie ermöglichen jedoch nicht eine sichere Pulsdiagnostik.
• Bei stark pigmentierter Haut kann es zu Messabweichungen kommen.
• Verwenden Sie das Pulsoximeter
- NICHT während einer MRT- oder CT-Untersuchung.
- NICHT während eines Patiententransports außerhalb einer medizinischen Einrichtung.
- NICHT an einem abgedrückten Anwendungsfinger oder Arm (bspw. Blutdruckmessung mittels
- NICHT an Fingern mit Nagellack, Beschmutzungen oder Pflasterverbänden.
- NICHT in der Nähe von brennbaren oder explosiven Gasgemischen.
- NICHT in Krankenhäusern in AP und APG Räumen.
Allgemeine Vorsichtsmaßnahmen
Bei Nichtbeachtung der nachfolgenden Anweisungen kann es zu fehlerhaften Messungen oder Mess-
• Auf dem Messfinger darf sich kein Nagellack, Kunstnagel oder andere Kosmetika befinden.
• Achten Sie beim Messfinger darauf, dass der Fingernagel so kurz ist, dass die Fingerbeere die
Sensorelemente im Gehäuse bedeckt.
• Halten Sie Hand, Finger und Körper während des Messvorgangs ruhig.
• Bei Personen mit Herzrythmusstörungen können die Messwerte von SpO und der Herzfrequenz
verfälscht sein oder die Messung ist gar nicht erst möglich.
• Bei Verwendung von Elektrochirurgiegeräten oder Defibrillatoren kann die Funktionalität des Puls-
oximeters beeinträchtigt werden.
• Das Pulsoximeter zeigt im Falle von Kohlenmonoxidvergiftungen zu hohe Messwerte an.
• Um das Messergebnis nicht zu verfälschen, sollte sich in der unmittelbaren Umgebung des Pulsoxi-
meters keine starke Lichtquelle (z.B. Leuchtstoampe oder direkte Sonneneinstrahlung) befinden.
• Bei Personen, die einen niedrigen Blutdruck haben, unter Gelbsucht leiden oder Medikamente zur
Gefäßkontraktion einnehmen, kann es zu fehlerhaften oder verfälschten Messungen kommen.
• Bei Patienten, denen in der Vergangenheit klinische Farbstoe verabreicht wurden und bei Pati-
enten mit abnormalem Hämoglobinvorkommen ist mit einer Messverfälschung zu rechnen. Dies
gilt insbesondere bei Kohlenmonoxidvergiftungen und Methämoglobinvergiftungen, welche z.B.
durch die Zugabe von Lokalanästhetika oder bei vorliegendem Methämoglobinreduktase-Mangel
• Bei Patienten mit arteriellem Katheter, Hypotonie, starken Gefäßverengungen, Blutarmut oder Un-
terkühlungen kann es zu Messversagen kommen.
• Verwenden Sie das Gerät unter den jeweils zulässigen Betriebs- und Lagerbedingungen.
• Schützen Sie das Pulsoximeter vor Staub, Erschütterungen, Nässe, extremen Temperaturen und
• Benutzen Sie das Gerät nicht in der Nähe von starken elektromagnetischen Feldern und halten Sie
es fern von Funkanlagen oder Mobiltelefonen.
• Bringen Sie das Gerät vor der Messung auf Raumtemperatur. Wenn das Gerät nahe der maximalen
oder minimalen Lager- und Transporttemperatur gelagert wurde und in eine Umgebung mit einer
Temperatur von 20 °C gebracht wird, wird empfohlen, vor Verwendung des Geräts ca. 4 Stunden
• Die Daten-Mittelwertbildung und die Signalverarbeitung führen zu einer Verzögerung bei der Ak-
-Werte. Wenn der Datenaktualisierungszeitraum weniger als 30 Sekunden
beträgt, verlängert sich die Zeit für die Ermittlung der dynamischen Durchschnittswerte, was auf
Signalverschlechterung, geringe Perfusion oder andere Störungen zurückzuführen ist.
• Funktionstester können verwendet werden, um zu überprüfen, ob das Gerät normal funktioniert,
z. B. Fluke INDEX-2LFE-Simulator, Fluke Index ProSim 8 Simulator. Die detaillierten Bedienungs-
schritte entnehmen Sie bitte dem Handbuch.
• Funktionstester können nicht verwendet werden, um die Genauigkeit eines Pulsoximeters zu be-
Hinweise zum Umgang mit Batterien
• Batterien immer korrekt und unter Berücksichtigung der Polaritäten (+ / -) einlegen. Batterien sau-
ber und trocken halten und von Wasser fernhalten. Stets den richtigen Batterietyp wählen.
• Batterien und Kontakte des Batteriefachs niemals kurzschließen.
• Batterien niemals aufladen, zwangsentladen, erhitzen, zerlegen, deformieren, einkapseln oder mo-
• Niemals an Batterien schweißen oder löten.
• Batterien unterschiedlicher Herstellung, Kapazität (neu und gebraucht), Größe und Typ innerhalb
eines Gerätes niemals mischen.
• Explosionsgefahr! Nichtbeachtung der genannten Punkte kann zu Personenschäden, Überhit-
zung, Auslaufen, Entlüftung, Bruch, Explosion oder Feuer führen.
• Wenn eine Batterie ausgelaufen ist, Schutzhandschuhe anziehen und das Batteriefach mit einem
• Wenn Flüssigkeit aus einer Batteriezelle mit Haut oder Augen in Kontakt kommt, die betroene
Stelle mit Wasser auswaschen und ärztliche Hilfe aufsuchen.
• Verschluckungsgefahr! Batterien außerhalb der Reichweite von Kindern aufbewahren. Bei Ver-
schlucken sofort ärztliche Hilfe aufsuchen. Das Verschlucken kann zu schweren inneren Verbren-
nungen und dem Tod führen.
• Niemals Kindern erlauben, Batterien ohne Aufsicht eines Erwachsenen auszutauschen.
• Batterien entfernt von Metallgegenständen, in gut belüfteten, trockenen und kühlen Räumen lagern.
• Batterien niemals direkter Sonneneinstrahlung oder Regen aussetzen.
• Werden Batterien einer Umgebung mit extrem hohen Temperaturen oder extrem niedrigem Luft-
druck ausgesetzt, kann dies zu einer Explosion oder zum Auslaufen von entflammbaren Flüssig-
• Bei längerer Nichtnutzung Batterien aus dem Gerät entfernen. Produkt nicht mehr nutzen, wenn die
Batteriefachabdeckung nicht mehr richtig schließt.
• Entladene Batterien sofort und ordnungsgemäß entsorgen. Batterien niemals im Feuer oder heißen
• Bei der Entsorgung, Batterien mit unterschiedlichen elektrochemischen Systemen getrennt auf-
Die Funktionstaste des Pulsoximeters hat folgende Funktionen:
• Einschalt-Funktion: Um das Pulsoximeter einzuschalten, Funktionstaste drücken.
• Helligkeits-Funktion: Um die Display-Helligkeit einzustellen, während des Betriebs Funktionstaste
gedrückt halten. Es kann zwischen fünf verschiedenen Helligkeitsstufen gewählt werden.
Die Ausrichtung der Display-Anzeige (Hochformat, Querformat) erfolgt automatisch. Dadurch
können Sie die Werte auf dem Display jederzeit gut ablesen, egal wie Sie das Pulsoximeter halten.
MESSERGEBNISSE BEURTEILEN
Die nachfolgende Tabelle zur Beurteilung Ihres Messergebnisses gilt NICHT für Personen mit be-
stimmten Vorerkrankungen (z.B. Asthma, Herzinsuzienz, Atemwegserkrankungen) und bei Aufent-
halten in Höhenlagen über 1500 Metern. Wenn Sie unter Vorerkrankungen leiden, wenden Sie sich
zur Beurteilung Ihrer Messwerte immer an Ihren Arzt.
Messergebnis SpO (Sauer-
Einstufung / Zu treende Maßnahmen
93-90 Erniedrigter Bereich: Arztbesuch empfohlen
<90 Kritischer Bereich: Dringend Arzt aufsuchen
Quelle: In Anlehnung an „Windisch W et al. S2k-Leitlinie: Nichtinvasive und invasive Beatmung als
Therapie der chronischen respiratorischen Insuzienz Revision 2017; Pneumologie 2017; 71: 722795“
Höhenabhängiger Sauerstosättigungsabfall
Die nachfolgende Tabelle informiert Sie über die Auswirkungen unterschiedlicher Höhenlagen
auf den Sauerstosättigungswert sowie deren Folgen für den menschlichen Organismus. Die
nachfolgende Tabelle gilt NICHT für Personen mit bestimmten Vorerkrankungen (z.B. Asthma,
Herzinsuzienz, Atemwegserkrankungen etc.). Bei Personen mit Vorerkrankungen können
Krankheitssymptome (z.B. Hypoxie) bereits in niedrigeren Höhenlagen auftreten.
(Sauerstoffsättigung) in %
1500-2500 m >90 Keine Höhenkrankheit (in der Regel)
2500-3500 m ~90 Höhenkrankheit, Anpassung empfohlen
Sehr häufiges Auftreten einer Hö-
henkrankheit, Anpassung zwingend
Schwere Hypoxie, nur zeitlich begrenzter
7500-8850 m <70 Sofortige akute Lebensgefahr
Quelle: Hackett PH, Roach RC: High-Altitude Medicine. In: Auerbach PS (ed): Wilderness Medicine, 3rd edition;
Mosby, St.Louis, MO 1995; 1-37.
REINIGUNG / INSTANDHALTUNG
Wenden Sie am Pulsoximeter keine Hochdruck- oder Ethylenoxid-Sterilisation an! Das Gerät ist nicht für
Sterilisationen geeignet. Halten Sie das Pulsoximeter auf keinen Fall unter Wasser, da sonst Flüssigkeit
eindringen kann und das Pulsoximeter beschädigt wird.
• Reinigen Sie nach jeder Anwendung das Gehäuse und die gummierte Innenfläche des Pulsoxime-
ters mit einem weichen, mit medizinischem Alkohol angefeuchteten Tuch.
• Wenn Sie das Pulsoximeter länger als einen Monat nicht benutzen, entnehmen Sie beide Batterien
aus dem Gerät, um ein eventuelles Auslaufen der Batterien zu verhindern.
Bewahren Sie das Pulsoximeter in einer trockenen Umgebung auf. Zu hohe Luftfeuchtigkeit kann die
Lebensdauer des Pulsoximeters verkürzen oder es beschädigen.
Im Interesse des Umweltschutzes darf das Gerät am Ende seiner Lebensdauer nicht mit dem
Hausmüll entsorgt werden. Die Entsorgung kann über entsprechende Sammelstellen in Ihrem
Land erfolgen. Entsorgen Sie das Gerät gemäß der Elektro- und Elektronik Altgeräte EG-Richtli-
nie – WEEE (Waste Electrical and Electronic Equipment). Bei Rückfragen wenden Sie sich an die
für die Entsorgung zuständige kommunale Behörde.
Batterien dürfen nicht über den Hausmüll entsorgt werden. Sie können giftige Schwermetalle enthalten
und unterliegen der Sondermüllbehandlung.
Diese Zeichen finden Sie auf schadstohaltigen Batterien:
Pb = Batterie enthält Blei,
Cd = Batterie enthält Cadmium,
Hg = Batterie enthält Quecksilber.
Problem Mögliche Ursache Behebung
korrekt in das Pulsoximeter
Legen Sie den Messfinger erneut in das
Der gemessene SpO ist zu
Messung erneut durchführen. Sollte das
Problem mehrmals auftauchen und das
Gerät in einwandfreiem Zustand sein, unbe-
dingt einen Arzt aufsuchen.
direkte Sonneneinstrahlung)
befindet sich in der Nähe.
Pulsoximeter von starken Lichtquellen
Die Batterien im Pulsoxime-
Tauschen Sie die Batterien aus.
Batterien erneut einlegen.
Das Pulsoximeter ist defekt.
Kontaktieren Sie Ihren Händler oder den
Warn- und Sicherheitshinsweise beachten.
Messfinger ist zu groß oder
Fingerspitze muss folgende Maße haben:
Breite zwischen 7-11 mm, Länge mind.
befindet sich in Bewegung.
Finger, Hand und Körper während der Mes-
Herzrythmusstörungen Einen Arzt aufsuchen.
Messmethode Nicht invasive Messung der arteriellen Sauerstosättigung des Hämo-
globins und Pulsfrequenz am Finger
Messbereich SpO 0 – 100 , %
Puls 30 – 250 Schläge /Minute
Genauigkeit SpO 70 100 %, ± 2 %, unter 70% nicht spezifiziert –
Puls ≤99 bpm +/- 2 bpm, ≥100 bpm +/- 2%
Abmessungen L 59 mm x B 37 mm x H 35 mm
Gewicht Ca. 27 g (ohne Batterien)
Rotlicht (Wellenlänge 660 nm), optische Ausgangsleistung < 6,65 mW;
Infrarot (Wellenlänge 905 nm), optische Ausgangsleistung < 6,75 mW;
Diese Informationen können für Kliniker besonders nützlich sein
+10 °C bis +40 °C, ≤ 75 % relative Luftfeuchte,
700 – 1060hPa Umgebungsdruck
-40 °C bis +60 °C, ≤ 95 % relative Luftfeuchte
Batterie-Lebensdauer 2 AAA Batterien ermöglichen ca. 2,5 Jahre Betrieb bei 3 Messungen
Information zur Lebensdauer des Produkts finden Sie auf der Home-
Klassifikation IP22, Anwendungsteil Typ BF
Die Seriennummer befindet sich auf dem Gerät oder im Batteriefach.
Änderungen der technischen Angaben ohne Benachrichtigung sind aus Aktualisierungsgründen vor-
• Dieses Gerät entspricht der europäischen Norm EN 60601-1-2 (Gruppe 1, Klasse B, in Überein-
stimmung mit CISPR 11, IEC 61000-4-2, IEC 61000-4-3, IEC 61000-4-8) und unterliegt besonderen
Vorsichtsmaßnahmen hinsichtlich der elektromagnetischen Verträglichkeit. Bitte beachten Sie da-
Lesen Sie diese Gebrauchsanweisung sorgfältig durch. Befolgen Sie die Warn-
und Sicherheitshinweise. Bewahren Sie die Gebrauchsanweisung für den spä-
teren Gebrauch auf. Machen Sie die Gebrauchsanweisung anderen Benutzern
zugänglich. Geben Sie bei Weitergabe des Geräts auch die Gebrauchsanweisung
PULSOXIMETER | PULSE OXIMETER
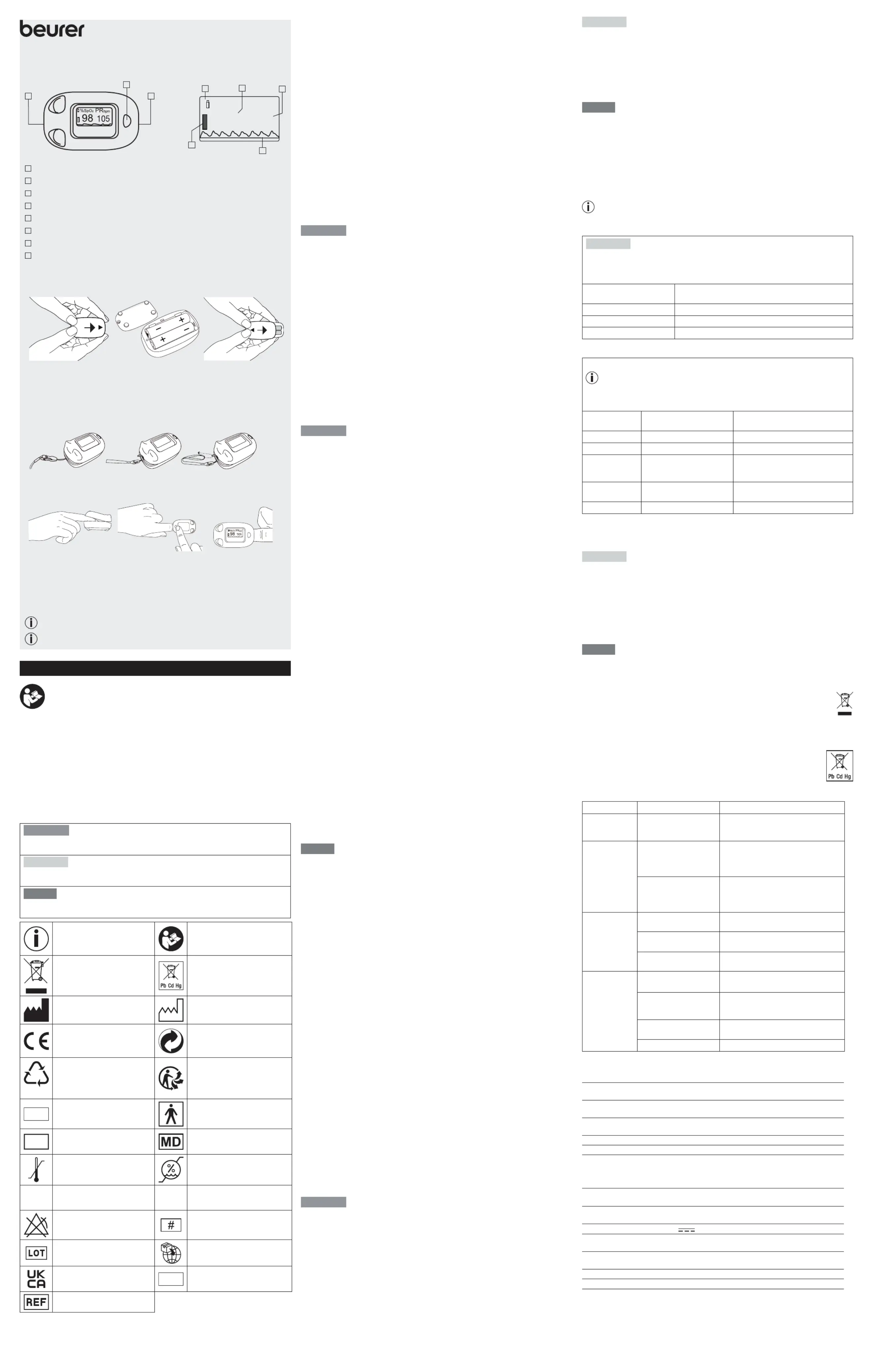
GERÄTEBESCHREIBUNG | DEVICE DESCRIPTION
Umhängeband-Halterung | Lanyard holder
Funktionstaste | unction button
Fingerönung | Finger opening
Sauerstosättigung (Wert in Prozent) | Oxygen saturation (value in percent)
Pulsfrequenz (Wert in Pulsschläge pro Minute) | Pulse rate (value in beats per minute)
Pulswelle (Plethysmografische Welle; normiert) | Pulse wave (plethysmographic wave; normalised)
Batterieanzeige | Battery indicator
Batterien einlegen | Inserting the batteries
Schraube mit Schraubendre-
her lösen | Batteriefach önen
screwdriver | Open the battery
Batterien mit korrekter Polung
Insert the batteries with the
Batteriefach mit Schraube
Close the battery compart-
ment | Secure the battery
compartment with the screw
Umhängeband befestigen | Attaching the lanyard
Inbetriebnahme | Initial use
Finger in Önung legen und
opening and hold it steady
Funktionstaste drücken und
während der Messung nicht
Press the function button
and do not move during the
Pulsoximeter schaltet sich wenige Sekunden nach Fingerentnahme aus.
Pulse oximeter switches o a few seconds after the person withdraws their finger.