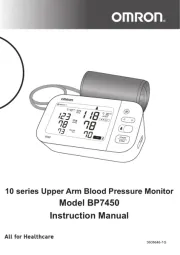
Wearable Blood Pressure Monitor
HeartGuide (HEM-6411T-MAE)
Read the instruction manuals before use.
Important safety information is in Instruction Manual
How to set up the wrist monitor is in Instruction Manual
IM1-HEM-6411T-MAE-03-09/2022
Thank you for purchasing the OMRON HEM-6411T-MAE Automatic Wrist Blood Pressure
Monitor. This blood pressure monitor is to be worn on your wrist. This blood pressure
monitor uses the oscillometric method of blood pressure measurement. When the band
in ates, the monitor senses the pressure pulsations of the artery underneath the band.
The pulses are called oscillometric pulses. The electronic pressure sensor displays a
digital reading of blood pressure.
This monitor can also track other measurements.
This monitor tracks steps and calculates calories burned and distance.
This monitor measures hours slept and awake time.
This instruction manual provides you with important information about the OMRON
HEM-6411T-MAE Automatic Wrist Blood Pressure Monitor. To ensure the safe and
proper use of your monitor, READ and UNDERSTAND all of the safety and operating
instructions. If you do not understand these instructions or have any questions, contact
your OMRON retail outlet or distributor before attempting to use this monitor. For
speci c information about your own blood pressure, consult with your physician.
The device is a digital monitor intended for use in measuring blood pressure and pulse
rate in adult patient population with wrist circumference ranging from 16 to 19 cm. This
monitor detects the appearance of irregular heartbeats during measurement and gives a
warning signal with readings. It is mainly designed for general household use.
1.3 Receiving and Inspection
Remove this monitor from the packaging and inspect for damage. If this monitor is
damaged, DO NOT USE and consult with your OMRON retail outlet or distributor.
a Display e Charging port
b [START/STOP] button f Cu
c [FORWARD] button g Band
Press this button from any screen to
To use your monitor comfortably, install the
cu sleeve to the cu of the monitor.
Guidelines for the management of arterial hypertension
De nitions of hypertension by o ce and home blood pressure levels
Systolic Blood Pressure ≥ 140 mmHg ≥ 135 mmHg
Diastolic Blood Pressure ≥ 90 mmHg ≥ 85 mmHg
These ranges are from statistical values for blood pressure.
* European Society of Hypertension (ESH) and European Society of Cardiology (ESC).
Blood pressure level indicator (color)
When your systolic or diastolic blood pressure is above the ESC/ESH Guidelines, the
number will be shown in red. Refer to “2018 ESH/ESC Guidelines for the management
of arterial hypertension” for more information.
3. Important Safety Information
Read the Important Safety Information in this instruction manual before using this
monitor. Follow this instruction manual thoroughly for your safety. Keep for future
reference. For speci c information about your own blood pressure, CONSULT WITH
Indicates a potentially hazardous situation which, if not avoided,
could result in death or serious injury.
• DO NOT use this monitor on infants, toddlers, children or persons who cannot express
• DO NOT adjust medication based on readings from this blood pressure monitor. Take
medication as prescribed by your physician. ONLY a physician is quali ed to diagnose
and treat high blood pressure.
• DO NOT use this monitor on an injured arm or an arm under medical treatment.
• DO NOT wear this monitor on your wrist while on an intravenous drip or blood
• DO NOT use this monitor in areas containing high frequency (HF) surgical equipment,
magnetic resonance imaging (MRI) equipment, computerized tomography (CT)
scanners. This may result in incorrect operation of the monitor and/or cause an
• DO NOT use this monitor in oxygen rich environments or near ammable gas.
• Consult with your physician before using this monitor if you have common
arrhythmias such as atrial or ventricular premature beats or atrial brillation; arterial
sclerosis; poor perfusion; diabetes; pregnancy; preeclampsia or renal disease. NOTE
that any of these conditions in addition to patient motion, trembling, or shivering may
a ect the blood pressure measurement reading.
• NEVER diagnose or treat yourself based on your readings. ALWAYS consult with your
• To help avoid strangulation, keep the AC adapter cable away from infants, toddlers or
• This product contains small parts that may cause a choking hazard if swallowed by
infants, toddlers or children.
• This product emits radio frequencies (RF) in the 2.4 GHz band. DO NOT use this
product in locations where RF is restricted, such as on an aircraft or in hospitals. Turn
o the Bluetooth® feature in this monitor, remove batteries and/or unplug the AC
AC Adapter Handling and Usage
• DO NOT use the AC adapter if the monitor or the AC adapter cable is damaged. If this
monitor or the AC adapter cable is damaged, turn o the power and unplug the AC
• Plug the AC adapter into the appropriate voltage outlet. DO NOT use in a multi-outlet plug.
• NEVER plug in or unplug the AC adapter from the electric outlet with wet hands.
• DO NOT disassemble or attempt to repair the AC adapter.
• Dry your hands before touching the AC Adapter, the charging clip and the the device
• Keep the AC adapter, the charging clip and the device charging electrodes dry at all times.
Rechargeable Battery Handling and Usage
• The rechargeable battery has been speci cally designed for this monitor. DO NOT use it in
• DO NOT recharge the rechargeable battery once it has been removed from this monitor.
• DO NOT dispose of the battery in a re.
• DO NOT crush or puncture the battery because it may cause spontaneous ames.
• DO NOT disassemble or modify the battery.
• DO NOT connect the + and – poles using a piece of metal or other conductive objects.
• DO NOT carry it or store it together with item such as necklaces and hair pins.
• DO NOT recharge, use, or leave the battery in any high temperature environment such as
in a location near a re or in direct sunlight. Doing so may cause the battery to overheat,
• DO NOT leave the battery that has been removed within the reach of infants, toddlers,
children or pets. Doing so may result in an injury or an accident. If liquid comes in contact
with the battery, a re or an accident may occur.
• This monitor has a built-in rechargeable battery. To prevent the risk of overheating, re
or explosion, DO NOT throw into re, apply heat, puncture or crush, use or leave in a high
Indicates a potentially hazardous situation which, if not avoided, may
result in minor or moderate injury to the user or patient or damage to
the equipment or other property.
• Stop using this monitor and consult with your physician if you experience skin irritation
• DO NOT use this device if you have a metal allergy.
• Keep this monitor clean. If this monitor is causing skin irritation due to sweat or
contamination, stop using it and consult with your dermatologist.
• Prolonged rubbing and pressure may irritate the skin. Give your wrist a break by
removing the monitor for a while after extended wear.
• DO NOT let chemical products such as lotions, oils, skin creams or cosmetics collect on the
band. To avoid damage to the band material, make sure to wipe o any chemical products
that have collected on the band.
• Consult with your physician before using this monitor on an arm where intravascular
access or therapy, or an arteriovenous (A-V) shunt, is present because of temporary
interference to blood ow and could result in injury.
• Consult with your physician before using this monitor if you have had a mastectomy
• Consult with your physician before using this monitor if you have severe blood ow
problems or blood disorders as cu in ation can cause bruising.
• DO NOT take measurements more often than necessary because bruising, due to
blood ow interference, may occur.
• ONLY in ate the cu when it is applied on your wrist.
• Remove the band if it does not start de ating during a blood pressure measurement.
• During measurement, make sure that no mobile device or any other electrical device
that emits electromagnetic elds is within 30 cm of this monitor. This may result in
incorrect operation of the monitor and/or cause an inaccurate reading.
• DO NOT disassemble or attempt to repair this monitor or other components. This may
cause an inaccurate reading.
• DO NOT use in a location where there is moisture or a risk of water splashing this
monitor. This may damage this monitor.
• The monitor and AC adapter are not designed for use in water or wet environments.
• DO NOT use this monitor in a moving vehicle such as in a car or on an aircraft.
• DO NOT drop or subject this monitor to strong shocks or vibrations.
• DO NOT use this monitor in places with high or low humidity or high or low temperatures.
• Ensure this monitor is not impairing blood circulation by observing the arm while
measurement is occurring.
• DO NOT use this monitor in high-use environments such as medical clinics or physician o ces.
• DO NOT use this monitor with other medical electrical (ME) equipment simultaneously.
This may result in incorrect operation of the monitor and/or cause an inaccurate reading.
• ONLY use this monitor on persons whose wrist circumference is within the speci ed
• Ensure that this monitor has acclimated to room temperature before taking a
measurement. Taking a measurement after an extreme temperature change could
lead to an inaccurate reading. OMRON recommends waiting for approximately 2 hours
for the monitor to warm up or cool down when the monitor is used in an environment
within the temperature speci ed as operating conditions after it is stored either at
the maximum or at the minimum storage temperature. For additional information of
operating and storage/transport temperature, refer to section 9.
• DO NOT use this monitor after the durable period has ended. Refer to section 9.
• DO NOT crease the band excessively.
• DO NOT use the blood pressure measuring function for any other purpose.
• DO NOT use this monitor to diagnose sleep disorders.
• Consult with your physician or healthcare provider if you begin a weight reduction or
• Keep the product out of the reach of infants, toddlers and children.
• Avoid bathing, drinking alcohol or ca eine, smoking, exercising and eating for at least
30 minutes before taking a measurement.
• Rest for at least 5 minutes before taking a measurement.
• Remove tight- tting, thick clothing and any accessories from your arm while taking a
blood pressure measurement.
• Remain still and DO NOT talk while taking a measurement.
AC Adapter Handling and Usage
• DO NOT plug the AC adapter cable into any device other than this monitor.
• Fully insert the AC adapter into the outlet.
• When unplugging the AC adapter from the outlet, be sure to safely pull from the AC
adapter. DO NOT pull from the AC adapter cable.
• When handling the AC adapter cable:
DO NOT damage it. / DO NOT break it. / DO NOT tamper with it. / DO NOT pinch it. /
DO NOT forcibly bend or pull it. / DO NOT twist it.
DO NOT use it if it is gathered in a bundle.
DO NOT place it under heavy objects.
• Wipe any dust or debris o the AC adapter.
• Unplug the AC adapter when not in use.
• Unplug the AC adapter before cleaning this monitor.
• ONLY use the AC adapter speci ed for this monitor. Use of unsupported AC adapters
may damage and/or may be hazardous to this monitor.
Rechargeable Battery Handling and Usage
• DO NOT charge the battery when the AC adapter is wet.
Press and hold the middle and lower button at the same time for more than 7 seconds
Continue to the back side
5. Error Messages and Troubleshooting
If any of the below problems occur during measurement, check to make sure that no other
electrical device is within 30 cm. If the problem persists, please refer to the table below.
Remove your monitor. Wait for 2 - 3 minutes
and then take another measurement. Repeat
the steps in Instruction Manual
continues to appear, we recommend that you
consult with your physician.
Carefully read and repeat the steps in
Adjust the height of your wrist following the heart
zone indicator. Refer to step 8
Due to di erences in individual size and
physique, this feature may not be helpful in
all cases. If you feel the position of the wrist
does NOT match your heart level, turn o this
feature and follow your judgment. To disable
this feature, refer to back side of Instruction
Charge the battery. Refer to step 2 of
Follow the instructions shown in the
“HeartAdvisor” app. If it still appears after checking
the app, contact your OMRON retail outlet or
distributor. Refer to “www.omron-healthcare.com”
Apply the band correctly, then take another
measurement. Refer to step 7 of Instruction
Contact your OMRON retail
outlet or distributor. Refer to
“www.omron-healthcare.com” for contact
Remove any clothing interfering with the
Apply the band correctly, then take another
measurement. Refer to step 7 of Instruction
Do not touch your monitor while taking a
Remain still and do not talk during a
Apply the band correctly, then take another
measurement. Refer to step 7 of Instruction
Remain still and sit correctly during a
Take another measurement making sure not to
The detailed user manual for the HeartGuide is now
available on the web. Scan the QR code below using
your smartphone or type the URL below to access our
https://www.omron-healthcare.com/manuals/heartguide
OMRON HEALTHCARE Co., Ltd.
53, Kunotsubo, Terado-cho, Muko, KYOTO, 617-0002 JAPAN
OMRON HEALTHCARE EUROPE B.V.
Scorpius 33, 2132 LR Hoofddorp, THE NETHERLANDS
Production facility OMRON HEALTHCARE Co., Ltd.
1855-370, Kubo-cho, Matsusaka-shi, Mie, 515-8503 Japan
Subsidiaries OMRON HEALTHCARE UK LTD.
Opal Drive, Fox Milne, Milton Keynes, MK15 0DG, UK
www.omron-healthcare.com/distributors
OMRON MEDIZINTECHNIK HANDELSGESELLSCHAFT mbH
www.omron-healthcare.com/distributors
www.omron-healthcare.com/distributors